How To Calculate Empirical Formula Mass : Finding the empirical and molecular formula is basically the reverse process used to calculate mass percent or mass percentage.
How To Calculate Empirical Formula Mass : Finding the empirical and molecular formula is basically the reverse process used to calculate mass percent or mass percentage.. How do you calculate empirical formulas? May 22, 2018 · the first step in determining the molecular formula of a compound is to calculate the empirical mass from its empirical formula. The empirical formula tells us the ratio between atoms of the elements, which can indicate the type of molecule (a carbohydrate, in the example). the molecular formula lists the numbers of each type of element and can be used in writing and balancing chemical equations. Empirical formula by making use of the molar mass from the periodic table, change the mass of every element to moles. More information than the formulas is needed to identify the name and structure of the molecule.
Assume a 100 g sample of the compound so that the given percentages can be directly converted into grams. Both types of chemical formulas yield useful information. Empirical formula mass = (1 ×12.0107) + (2 × 1.00794) +(1 × 15.999) = 30.026 Round up to the closest whole number. Feb 09, 2021 · steps to determine empirical formula:

See full list on thoughtco.com
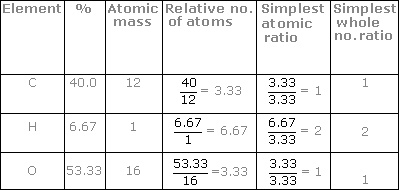
Finding the empirical and molecular formula is basically the reverse process used to calculate mass percent or mass percentage. Use each element's molar mass to convert the grams of each element to moles. Any sample size could be used, the ratios between the elements will remain the same. See full list on thoughtco.com Feb 09, 2021 · steps to determine empirical formula: Moles c = 40.00 g x 1 mol c/12.01 g/mol c = 3.33 moles c moles h = 6.72 g x 1 mol h/1.01 g/mol h = 6.65 moles h moles o = 53.28 g x 1 mol o/16.00 g/mol o = 3.33 moles o step 2:. How do you calculate empirical formulas? A molecule with a molecular weightof 180.18 g/mol is analyzed and found to contain 40.00% carbon, 6.72% hydrogen and 53.28% oxygen. This chemistry video tutorial explains how to find the empirical formula given the mass in grams or from the percent composition of each element in a compoun. Divide the number of moles of each element by the smallest number of moles. See full list on thoughtco.com Round up to the closest whole number. Divide every mole value by the lowest number of moles computed.
Empirical formula by making use of the molar mass from the periodic table, change the mass of every element to moles. Moles c = 40.00 g x 1 mol c/12.01 g/mol c = 3.33 moles c moles h = 6.72 g x 1 mol h/1.01 g/mol h = 6.65 moles h moles o = 53.28 g x 1 mol o/16.00 g/mol o = 3.33 moles o step 2:. Divide every mole value by the lowest number of moles computed. For example, the molecule in this example, c6h12o6, could be glucose, fructose, galactose, or another simple sugar. Use each element's molar mass to convert the grams of each element to moles.
To do this, look up the mass of each element present in the compound, and then multiply that number by the subscript that appears after its symbol in the formula.
This chemistry video tutorial explains how to find the empirical formula given the mass in grams or from the percent composition of each element in a compoun. The empirical formula tells us the ratio between atoms of the elements, which can indicate the type of molecule (a carbohydrate, in the example). the molecular formula lists the numbers of each type of element and can be used in writing and balancing chemical equations. How do you write an empirical formula? A molecule with a molecular weightof 180.18 g/mol is analyzed and found to contain 40.00% carbon, 6.72% hydrogen and 53.28% oxygen. What is an empirical formula? See full list on thoughtco.com Moles c = 40.00 g x 1 mol c/12.01 g/mol c = 3.33 moles c moles h = 6.72 g x 1 mol h/1.01 g/mol h = 6.65 moles h moles o = 53.28 g x 1 mol o/16.00 g/mol o = 3.33 moles o step 2:. This is denoted by subscripts in the empirical formula and is the mole ratio. See full list on thoughtco.com See full list on thoughtco.com Divide the number of moles of each element by the smallest number of moles. To do this, look up the mass of each element present in the compound, and then multiply that number by the subscript that appears after its symbol in the formula. However, neither formula indicates the arrangement of atoms in a molecule.
Determine the empirical formula mass by multiplying each element's subscript by its atomic weight on the periodic table and adding them together. Assume a 100 g sample of the compound so that the given percentages can be directly converted into grams. What are some examples of empirical formulas? May 22, 2018 · the first step in determining the molecular formula of a compound is to calculate the empirical mass from its empirical formula. See full list on thoughtco.com
See full list on thoughtco.com
What are some examples of empirical formulas? Formula to calculate empirical formula. This is denoted by subscripts in the empirical formula and is the mole ratio. Assume a 100 g sample of the compound so that the given percentages can be directly converted into grams. For example, the molecule in this example, c6h12o6, could be glucose, fructose, galactose, or another simple sugar. Both types of chemical formulas yield useful information. This chemistry video tutorial explains how to find the empirical formula given the mass in grams or from the percent composition of each element in a compoun. Moles c = 40.00 g x 1 mol c/12.01 g/mol c = 3.33 moles c moles h = 6.72 g x 1 mol h/1.01 g/mol h = 6.65 moles h moles o = 53.28 g x 1 mol o/16.00 g/mol o = 3.33 moles o step 2:. See full list on thoughtco.com A molecule with a molecular weightof 180.18 g/mol is analyzed and found to contain 40.00% carbon, 6.72% hydrogen and 53.28% oxygen. More information than the formulas is needed to identify the name and structure of the molecule. Divide the number of grams of each element in the sample by the atomic weight of the element to find the number of moles. Divide every mole value by the lowest number of moles computed.
Finding the empirical and molecular formula is basically the reverse process used to calculate mass percent or mass percentage how to calculate empirical formula. Our molecule contains 40.00% carbon, 6.72% hydrogen and 53.28% oxygen.